Production/Logistics/
Quality assurance/
Safety management
- Production
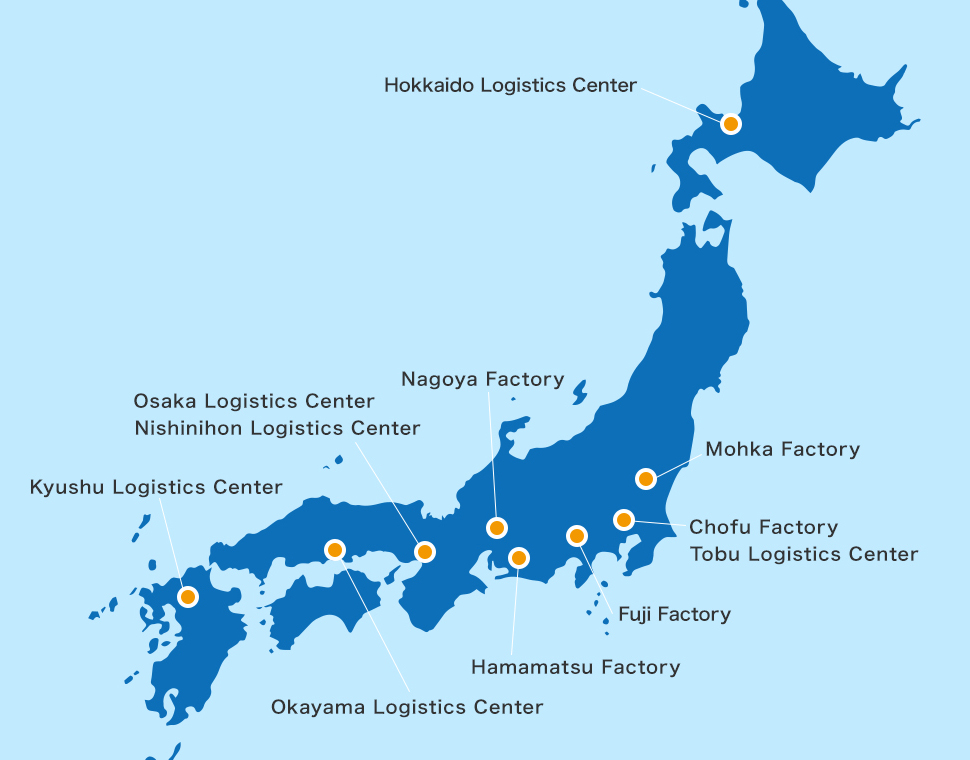
- Kowa owns manufacturing plants in Nagoya, Fuji, Hamamatsu, Mohka and Chofu for the production of its OTC, quasi-drugs, prescription drugs and medical devices.
- Logistics
- We have established logistics centers throughout Japan.
We are building a delivery system that uses cutting-edge technology,
including work automation within our logistics centers.
- Quality assurance
- We maintain an effective production management system in compliance with the Good Quality Practice (GQP) for pharmaceutical products to assure the high quality and reliability of our products.
Taking advantage of our experiences in pharmaceuticals, we are making our efforts, on dietary supplements, to construct a production and quality management on the manufacturing plants which meet Good Manufacturing Practice (GMP) for dietary supplements. This assures the high quality and reliability of our products.
- Safety management
- We have built and continue to maintain an effective safety management system in compliance with the Good Vigilance Practice (GVP), which allows us to collect information on adverse drug reactions from around the world. We then carefully evaluate and review this information to ensure that our products are used correctly, and immediately take safety measures if required.

 General Trading Business Unit
General Trading Business Unit Lifestyle Business Unit
Lifestyle Business Unit Pharmaceutical Business Unit
Pharmaceutical Business Unit Hospitality Business Unit
Hospitality Business Unit





